SARS-COV-2 Nucleocapsid (N) Antigen Rapid Detection Kit developed by Nanjing Synthgene Medical was successfully registered with the National Medical Products Administration of Ukraine
Release time:
2021-10-29
SARS-COV-2 Nucleocapsid (N) Antigen Rapid Detection Kit developed by Nanjing Synthgene Medical was successfully registered with the National Medical Products Administration of Ukraine.

SARS-COV-2 Nucleocapsid (N) Antigen Rapid Detection Kit developed by Nanjing Synthgene Medical was successfully registered with the National Medical Products Administration of Ukraine.
On November 29, SARS-COV-2 Nucleocapsid (N) Antigen Rapid Detection Kit(Colloidal gold method) developed by Nanjing Synthgene Medical was successfully registered with the National Medical Products Administration of Ukraine.
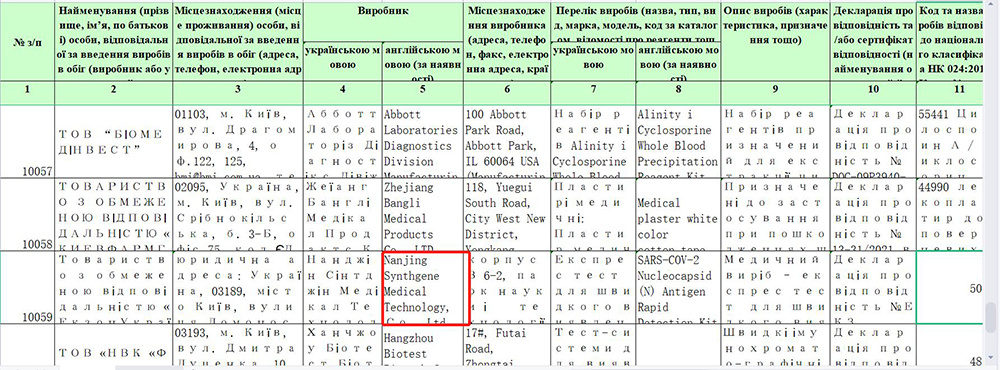
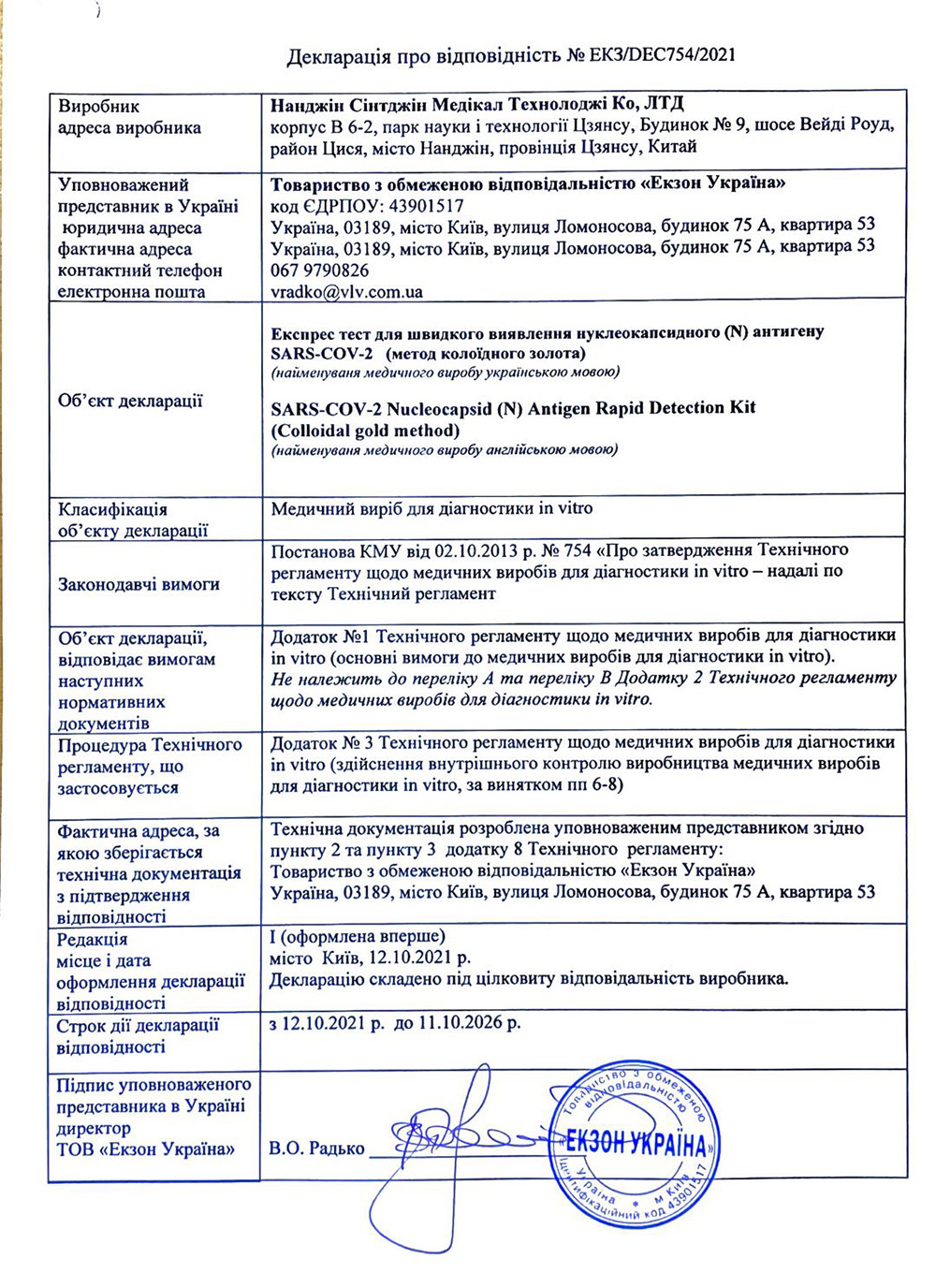
SARS-COV-2 Nucleocapsid (N) Antigen Rapid Detection Kit(Colloidal gold method) developed by Nanjing Synthgene Medical was successfully registered with the National Medical Products Administration of Ukraine, which means Nanjing Synthgene Medical’ SARS-COV-2 Nucleocapsid (N) antigen kit can be sold on the Ukrainian market, and Ukrainian citizens can purchase the product through supermarkets or pharmacies for self-testing, which will provide strong support for local epidemic prevention and control.
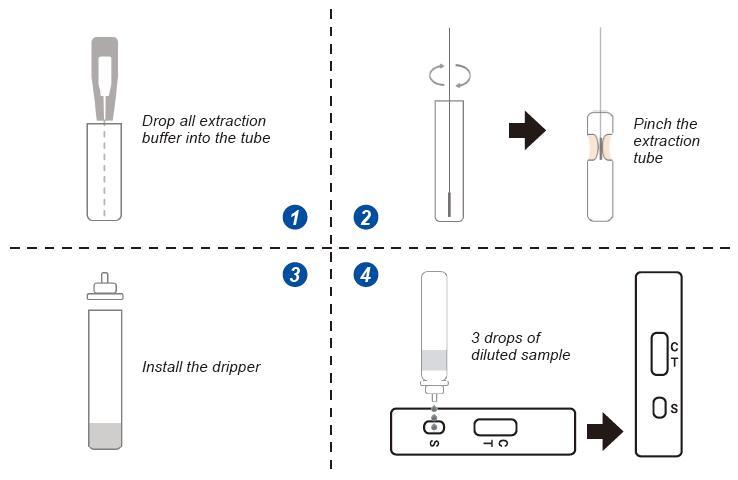
SARS-COV-2 Nucleocapsid(N) Antigen Rapid Detection Kit (Colloidal gold method) is used to detect the novel coronavirus antigen in human nasal swab samples, and is used to detect the Delta strain and other global epidemic strains, and the results can be obtained in 10 minutes.
Previously, the product has obtained the European Union CE, Japan, Iran, Lithuania, Indonesia and other countries and regions access qualifications, and has contributed a solid force to the global COVID-19 epidemic prevention and control. At the same time, it has won orders from many governments and won unanimous praise from overseas customers!
Features:
²1.Convenient sampling (non-invasive sample collection is convenient for individuals and
families to independently detect the COVID-19 virus)
²2. Simple operation (convenient operation, no need for professional equipment)
3. Fast detection (test results can be obtained quickly in 10 minutes)
4. Accurate results (highly accurate, comparable to PCR detection)
About the State Drug Administration of Ukraine
The National Medical Products Administration of Ukraine is the central executive agency of Ukraine. It was established on September 10, 2014 in accordance with Cabinet Resolution No. 442, which merged the National Medical Products Administration and the National Medical Products Administration. The National Medical Products Administration was established to ensure the implementation of national policies in the following areas: quality control and safety of drugs, including medical immunobiological drugs, medical equipment, and medical devices in circulation and/or in the field of protection, allowing them to be used in pharmacies Sales of its structural units; economic activity licensing for drug production and drug import, drug wholesale and retail trade; trafficking in narcotic drugs, psychotropic substances and their analogues and precursors, and combating illegal trafficking. The activities of this agency are directed and coordinated directly by the Cabinet of Ministers of Ukraine or through the Ministry of Health.


