Good News|Monkeypox Virus Detection Series Products developed by Nanjing Synthgene Medical have been registered with the British MHRA
Release time:
2022-09-02
Nearly 7,500 new monkeypox cases were reported globally last week, a 20 percent increase from the previous week, with almost all of the cases in Europe and the Americas, according to the World Health Organization.
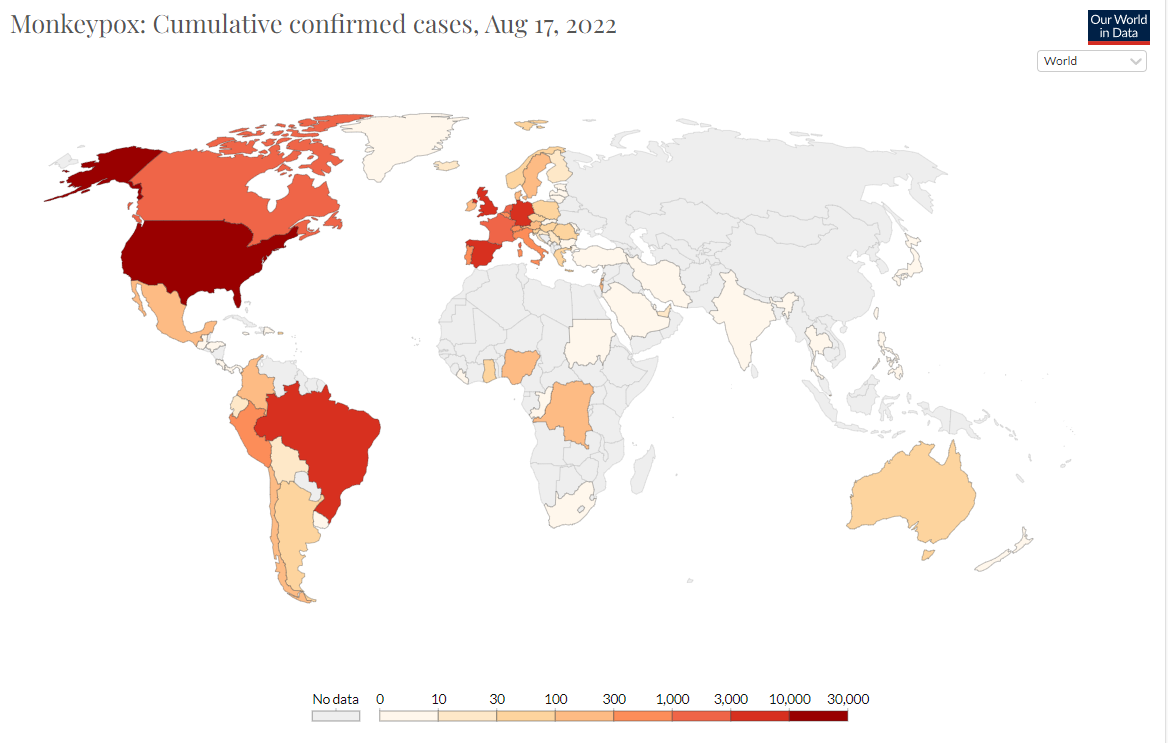
Nearly 7,500 new monkeypox cases were reported globally last week, a 20 percent increase from the previous week, with almost all of the cases in Europe and the Americas, according to the World Health Organization.
Tedros Adhanom Ghebreyesus, the Director-General of WHO,pointed out that there have been more than 35,000 cases of monkeypox, including 12 deaths, reported in 92 countries and regions around the world.The data also shows that the global monkeypox vaccine supply is still limited, and the relevant vaccine effectiveness data is also limited.
Tedros emphasized that it is important for all countries to develop and provide targeted services and information to target groups to protect their health, human rights and dignity. All countries need to prepare for monkeypox outbreaks with effective public health tools to stop transmission, enhanced disease surveillance, contact tracing, risk communication, and more.
Recently, three products developed by Nanjing Synthgene Medical have been registered with the British MHRA at the same time. The three products are "Monkeypox virus nucleic acid detection kit (RT-PCR method)", monkeypox virus antigen detection kit (colloidal gold method), monkeypox virus antibody detection kit (colloidal gold method). After these products have been registered with the British MHRA, they can be sold in the United Kingdom and countries that recognize the British MHRA registration.

MHRA is the abbreviation of the British Medicines Regulatory Agency, the full name of the Medicines and Healthcare products Regulatory Agency. MHRA is an executive government agency under the UK Department of Health that ensures the safety and efficacy of medicines and medical devices. After the United Kingdom leaves the European Union, according to the Brexit agreement, the EU CE certification will gradually no longer be recognized. For medical devices, the CE certification can continue to be used in the UK until June 30, 2023, but companies that need to hold CE certification have a local presence in the UK. The UK responsible person (similar to the EU authorized representative) is registered by the UK responsible person for MHRA to enter the UK GB market (England, Wales and Scotland). From July 1, 2023, CE certification will no longer be recognized, and UKCA certification must be carried out.
Previously, these three products developed by Synthgene Medical have obtained EU CE certificates, which means that these kits can be sold in EU countries and countries that recognize EU CE certification. The British MHRA registration of this series of products was approved again at the same time, which multi-dimensionally proves the excellent product performance and good user experience of the monkeypox virus detection reagent of Synthgene Medical.Synthgene Medical will make persistent efforts to contribute to epidemic prevention and control!

Related news
Related Products
undefined

