Wonderful and diverse丨Three exhibitions linked, with you to go to the international medical event
Release time:
2022-10-13
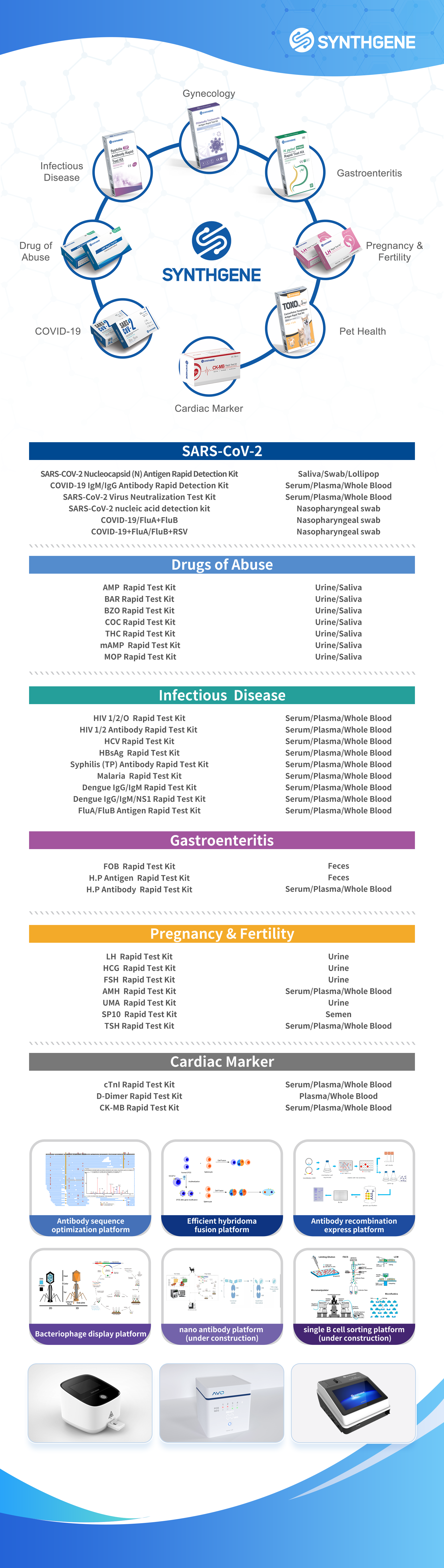
The COVID-19 pandemic is still continuing, and the medical industry at home and abroad is constantly seeking new breakthroughs. Synthgene is a biomedical high-tech enterprise focusing on the research and development and production of OTC home self-inspection products. The company has a mature technology platform, including chromatography platform, isothermal amplification platform, etc., the product line covers infectious disease detection, pet disease diagnosis, pregnancy diagnosis, drug detection, gastrointestinal function testing and other fields.

The COVID-19 pandemic is still continuing, and the medical industry at home and abroad is constantly seeking new breakthroughs. Synthgene is a biomedical high-tech enterprise focusing on the research and development and production of OTC home self-inspection products. The company has a mature technology platform, including chromatography platform, isothermal amplification platform, etc., the product line covers infectious disease detection, pet disease diagnosis, pregnancy diagnosis, drug detection, gastrointestinal function testing and other fields.
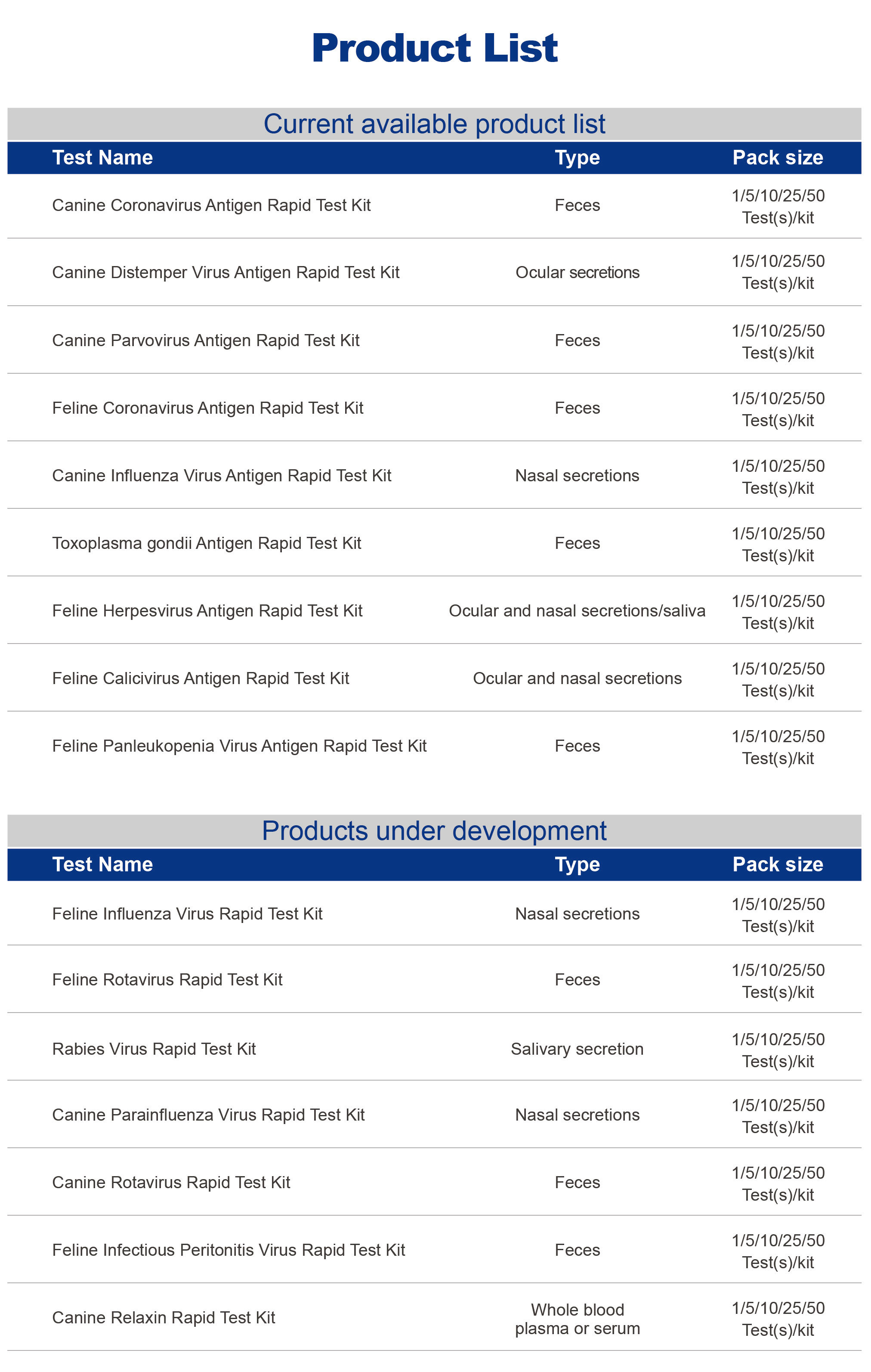
Synthgene's pet in vitro diagnostic reagents, to the quality requirements of human in vitro diagnostic reagents to develop and produce pet diagnostic reagents, we take innovation as the core, with technology to improve the pet medical diagnosis industry. We strictly control the quality and provide the most reliable products. Products are widely used in immunodiagnosis, molecular diagnosis, routine clinical testing and other fields, the main products of canine/cat toxoplasmosis kit, canine distemper kit has been exported to Hong Kong, Southeast Asia, Europe and North America.
Synthgene sincerely invites you to visit our booth to discuss and seek common development!

Exhibition Name: Singapore VET
Exhibition time: October 14, 2022 ~ October 15, 2022
Exhibition venue: Singapore· Suntec Singapore Convention & Exhibition Centre
Booth number: E10
Exhibition 2:Medlab Asia & Asia Health 2022

Exhibition Name::Medlab Asia & Asia Health 2022
Exhibition time:October 19 ~ October 21, 2022
Exhibition venue:Bangkok, Thailand· IMPACT Exhibition Center
Booth number:5.D16
Exhibitio3:Petfair SE ASIA 2022

Exhibition Name::Petfair SE ASIA 2022
Exhibition time:October 26 ~ October 28, 2022
Exhibition venue:Bangkok International Trade & Exhibition Centre)
Booth number:J8
Exhibits-IVD product line
Relying on the production line of immunodiagnostic raw materials, we have developed and mass-produced a variety of SARS-CoV-2 antigen antibody detection reagents, and the products have obtained the EU CE self-test certification and the export whitelist of the Ministry of Commerce, and have obtained access qualifications in Thailand, the Philippines, Germany, France, Belgium, Ukraine, the Netherlands, Spain, Japan, Peru and other countries and regions.
At the same time, the SARS-CoV-2 antigen detection kit (colloidal gold method) developed by Nanjing Synthgene was approved by the State Food and Drug Administration (registration certificate number: National Machinery Injection 20223400427) and officially listed, helping the rapid screening of the SARS-CoV-2 virus and contributing to the SARS-CoV-2 epidemic!
Now, the OTC home self-test product line has covered infectious disease testing, drug testing, women's health and other fields. At the same time, it provides downstream IVD enterprises with overall solutions for product research and development, production and registration.
Synthgene's SARS-CoV-2 antigen detection reagent related qualification display

Exhibits-Pet product line
Synthgene's pet in vitro diagnostic reagents, to the quality requirements of human in vitro diagnostic reagents to develop and produce pet diagnostic reagents, we take innovation as the core, with technology to improve the pet medical diagnosis industry. We strictly control the quality and provide the most reliable products. Products are widely used in immunodiagnosis, molecular diagnosis, routine clinical testing and other fields, the main products of canine/cat toxoplasmosis kit, canine distemper kit has been exported to Hong Kong, Southeast Asia, Europe and North America.


Related news
Related Products
undefined

