Hand in hand, a win-win new situation | SYNTHGENE Pharma Medlab Asia 2024 ended successfully!
Release time:
2024-07-16
Asia Health 2024 & Medlab Asia 2024 (Asia Health 2024 & Medlab Asia 2024) came to a successful conclusion in Bangkok, Thailand· on July 10-12.
Medical device manufacturers and industry experts from more than 50 countries around the world participated in the event to witness the new situation of the wave of medical devices going overseas!
As a leader in the diagnostic industry, SYNTHGENE Pharma has participated in the exhibition for many years, and exhibited a series of in vitro diagnostic products at this exhibition, demonstrating its importance to the Southeast Asian market and its competitiveness in the field of diagnostics.


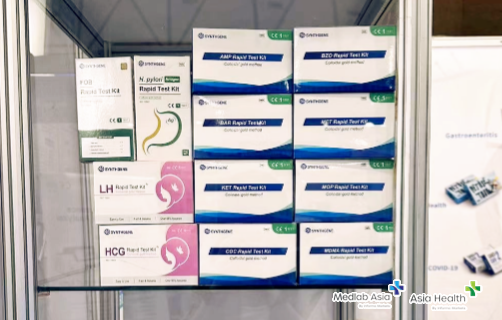

At the exhibition, the popularity of our booth continued to grow. Many foreign customers have come to hear about our professional technical strength and high-quality after-sales service, and our team's professional introduction and warm reception have won unanimous praise from customers.




The professional and technical personnel ofSYNTHGENE warmly received every guest with sincere and professional service, and introduced the product characteristics, development difficulties, differentiated advantages, and practical problems that can be solved in detail, which attracted the warm attention of many experts and customers in the industry, and came to the booth for in-depth discussions and exchanges, and highly affirmed the R&D capabilities and innovation value of SYNTHGENE.

Through in-depth exchanges and discussions, we have reached a cooperative relationship with some local customers in Thailand.
This not only marks the recognition of our products and technologies in the international market, but also indicates that we will carry out deeper business cooperation in Thailand and the wider region.
We will continue to cultivate at home and abroad to build hard core power
SYNTHGENE has always adhered to the development strategy of globalization, and currently has registered patents and trademarks at home and abroad. The products have obtained CE, EUA, and China NMPA certifications. SYNTHGENE solutions have served more than 200 countries and regions around the world, such as China, Europe, America, Asia, Australia, Africa, etc., providing efficient and professional customized services for global customers and winning the trust of global customers.

——International market access qualifications for in vitro diagnostic solutions

As the company's product line becomes more and more complete, product quality and service continue to improve, and overseas customers recognize us more and more. In the future, SYNTHGENE Pharma will strengthen cooperation with international customers, jointly promote the development of the medical industry, and provide more comprehensive laboratory solutions for global customers.

Related news
Related Products
undefined

