Exciting Update: Synthgene's Self-Test Products Achieve EU IVDR Certification!
Release time:
2025-04-17
Synthgene Biotech Obtains CE IVDR Certification for Self-Testing Kits: Reliable, Accurate & Easy-to-Use At-Home Diagnostics
Why is CE IVDR Certification Critical?
The EU's IVDR (In Vitro Diagnostic Regulation) sets the highest standards for safety and performance in self-testing diagnostics. Synthgene's Fecal Occult Blood (FOB), Syphilis (TP) Antibody, and H. pylori (HP) Antigen test kits HCG test kit have passed rigorous clinical evaluations, proving their accuracy and reliability for global users.
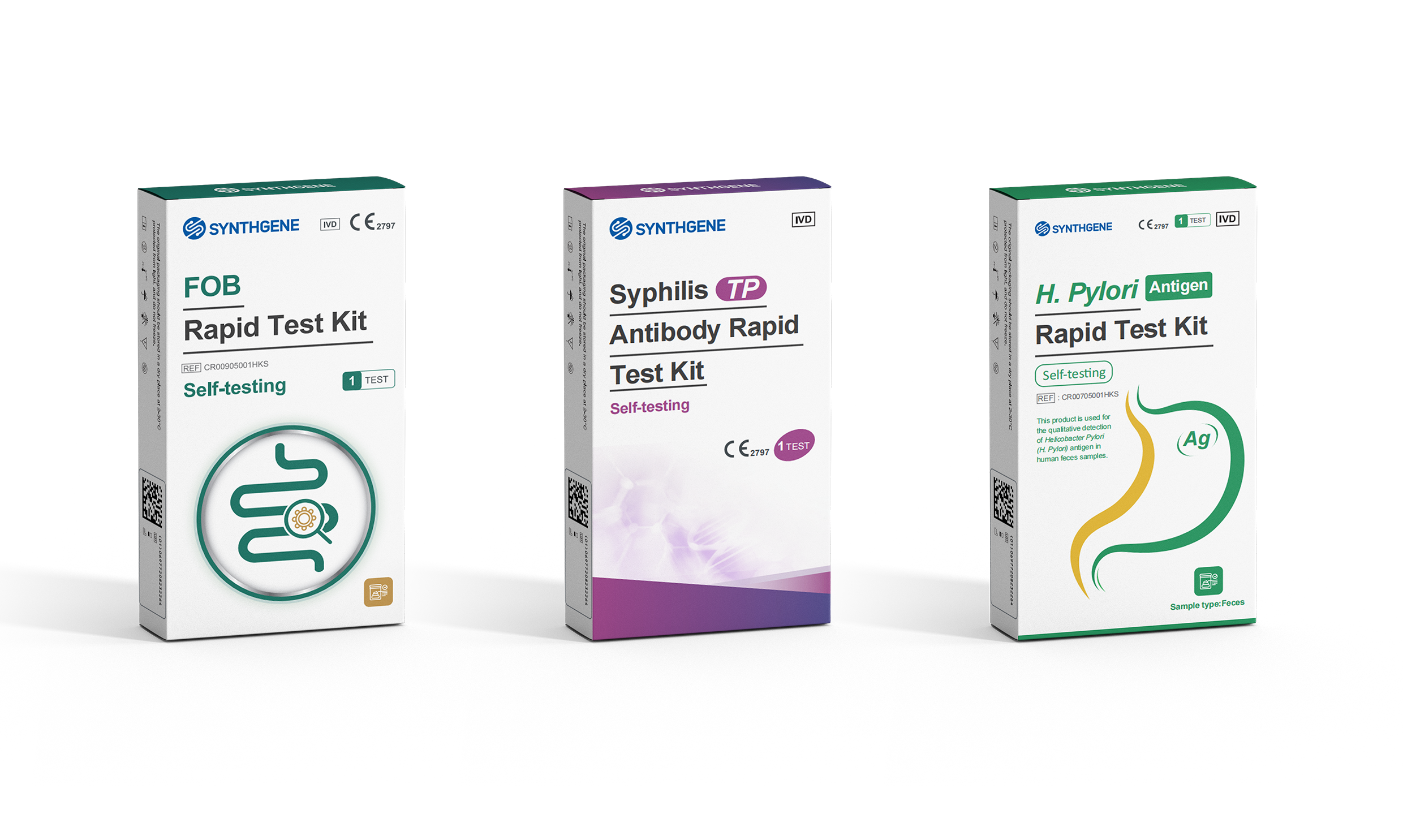
Key Advantages:
✔ Lab-Grade Accuracy – Highly consistent with professional lab results
✔ Effortless Self-Testing – No medical expertise required
✔ Universal Use – Suitable for adults & children
How It Works?
✔Collect – Easy self-sampling
✔Process – Follow simple instructions
✔Read – Clear positive/negative results in 10 minutes

Wide Availability – Get Tested Anytime, Anywhere
Synthgene’s self-test kits are conveniently available across multiple channels, ensuring easy access for every user:
- Pharmacies (e.g., DM in Germany, Boots in UK)
- Supermarkets & Convenience Stores
- E-commerce (Amazon EU & local platforms)
- Healthcare Facilities (clinics, hospitals)
- Vending Machines (airports, universities)
Whether you need quick at-home testing or professional backup, Synthgene delivers fast, accurate, and confidential solutions wherever you are.

This Class C Self-Testing Certification confirms Synthgene's compliance with the EU's strictest quality and safety standards, reinforcing our commitment to innovative, accessible diagnostics for homes, schools, and clinics.
Innovation Never Stops – Synthgene keeps advancing IVD technology to deliver better healthcare solutions worldwide.
Related news
Related Products
undefined

