SARS-CoV-2 Nucleocapsid (N) Antigen Rapid Detection Kit obtained EU CE self-test certificate
Release time:
2022-05-20
At present, the SARS-CoV-2-related testing products independently developed and produced by Synthgene have been sold in more than 30 overseas countries, and were approved by the State Food and Drug Administration on April 1, 2022 (Registration Certificate No.: National Machinery Note 20223400427), officially launched!
On May 20, 2022, the "SARS-COV-2 Nucleocapsid(N) Antigen Rapid Detection Kit (Colloidal gold method)" independently developed by Synthgene officially obtained the EU CE self-test certification!


This CE certification means that Synthgene Pharmaceutical's home self-test version of the SARS-CoV-2 antigen test kit can be sold in the 27 EU member states and other countries that recognize the EU CE certification, and consumers can buy it in major supermarkets or pharmacies and other channels , individuals can carry out testing operations, which not only saves testing time, but also meets the epidemic prevention needs of home SARS-CoV-2 testing, which will bring great convenience to ordinary people. In this regard, the company also stated that the certification of the testing product will help further enhance the international competitiveness of the company's SARS-CoV-2 testing products, expand the overseas application scenarios of the company's SARS-CoV-2 testing products, and will have a positive impact on the company's future operations.
Previously, Synthgene's SARS-CoV-2 test series products have been certified and recognized by many international authoritative institutions and organizations: On January 21, 2022, Synthgene's new coronavirus antigen detection kit SARS-COV-2 Nucleocapsid(N) Antigen Rapid Detection Kit (Colloidal gold method) successfully passed the review of the expert group of the EU Health Safety Committee and entered the whitelist of the HSC common list. The testing devices in this list are products with high sensitivity and specificity further screened by the EU Health and Safety Committee from the products that have obtained EU CE certification. Being able to be included in the EU HSC common list indicates that the analysis and clinical performance of Synthgene's testing products are fully recognized by the EU Health and Safety Committee.

In addition, the product has obtained access qualifications in Germany, France, Portugal, Ukraine, the Netherlands, Spain, Lithuania, Japan, Malaysia, Indonesia, Belgium and other countries and regions, and entered the Phase 2 evaluation of the NIBSC organization in the UK! In terms of product performance, thanks to Synthgene's superior R&D capabilities, its SARS-CoV-2 product R&D design still maintains excellent R&D capabilities. Synthgene SARS-CoV-2 products have covered a variety of product types from antigens, antibodies to neutralizing antibodies. From a technical point of view, there are colloidal gold method, quantum dot immunofluorescence chromatography, PCR and other methodological products, which can Fully adapt to the needs of anti-epidemic and scientific research in all regions of the world.
At present, the SARS-CoV-2-related testing products independently developed and produced by Synthgene have been sold in more than 30 countries overseas, and were approved by the State Food and Drug Administration on April 1, 2022 (Registration Certificate No.: National Machinery Note 20223400427), officially launched! After multiple certifications by authoritative organizations in various countries, the multi-dimensional proof of the excellent product performance and good user experience of Synthgene's new coronavirus antigen detection reagent. Our company will make persistent efforts to help the national and international response to the epidemic prevention and control work, and contribute to the epidemic prevention and control!
Nearly 100 kinds of products have obtained EU CE certification
Nearly 100 kinds of products independently developed by Synthgene have obtained EU CE certification. CE certification marks that our products meet the requirements expressed by a series of European directives such as safety, hygiene, environmental protection and consumer protection, which provides stronger support and guarantee for Synthgene's development and consolidation of foreign markets.



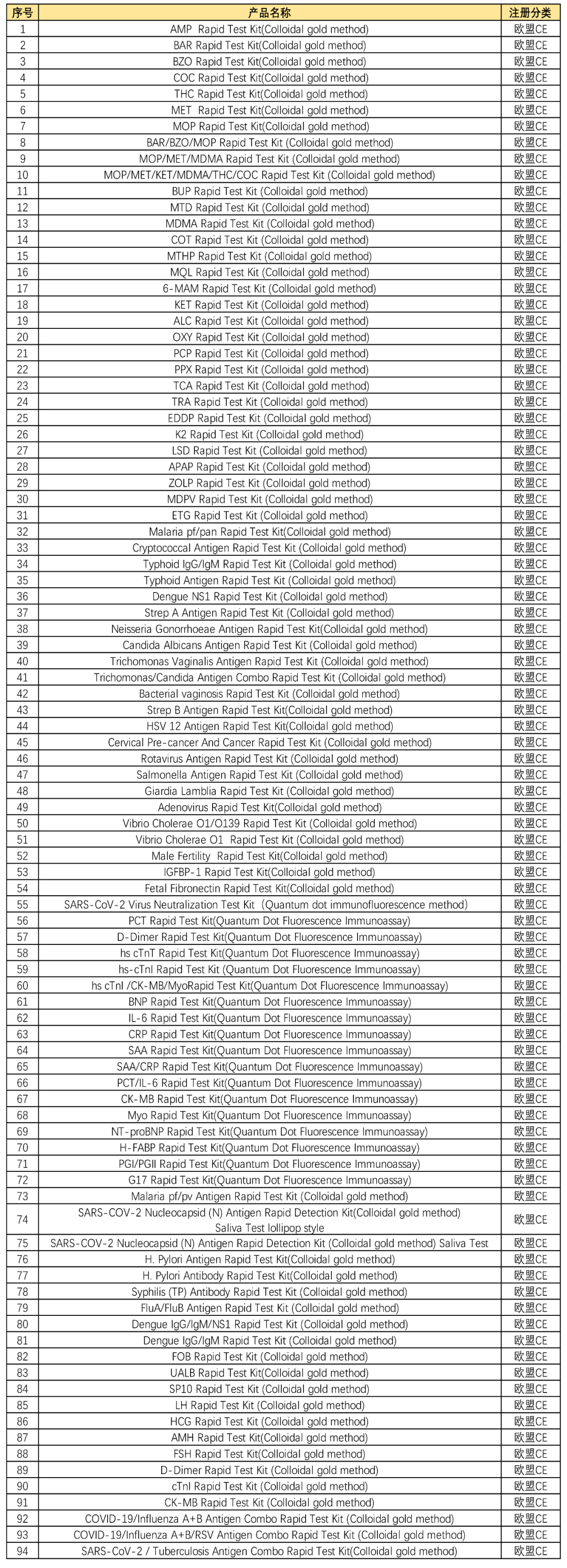
The above table is a list of approved product names, which covers infectious disease testing, drug testing, women's health and other fields. Passing CE certification means that nearly 100 products developed bySynthgene can be recognized in the 27 EU member states and other countries. EU CE certified countries listed on sale! The certification of testing products will help to further enhance the international competitiveness of the company's in vitro diagnostic testing products, broaden the overseas application scenarios of the company's in vitro diagnostic testing products, and effectively promote the company's rapid development.
Related news
Related Products
undefined

