cTnI Rapid Test Kit

The cardiac troponin test detects cardiac troponin I in whole humans/livestock/livestock in 10-15 minutes.
Test Type:whole blood 、 plasma 、serum
Detection time: 10-15 minutes
Ordering Information
Specifications and performance
|
Test Type: |
whole blood 、 plasma 、serum |
|
Detection time: |
10-15 minutes |
|
Reliability: |
≥99% |
|
Manufacturer: |
Nanjing Synthgene Medical Technology Co., Ltd. |
About this test
This kit is used for clinical in vitro qualitative detection of cardiac troponin I (cTnI) in human serum/plasma/whole blood (fingertip blood/venous blood). For professional use only. Troponin (trporin, Tn) is a structural protein that constitutes striated muscle filaments, and its subunits I, T and C form a complex. The content of cTnI in normal blood is very low. After myocardial injury, the cardiac troponin complex is released into the blood. After 4 to 6 hours, the elevated cTnI can be detected in the blood, which remains in the blood for 6 to 10 days, providing a longer detection period. cTnI has high myocardial specificity and sensitivity, so it has become the "gold standard" for acute myocardial infarction (AMI). The clinical significance of cTnI detection lies in early diagnosis and risk stratification of AMI, assessment of myocardial ischemic injury area and clinical treatment effect, auxiliary diagnosis of myocardial injury diseases caused by various reasons
The product only requires 3 drops of core/plasma or 2 drops of whole blood. If it is 3 drops of core/plasma, no sample solution is added. If it is 2 drops of whole blood, add 1 drop of sample solution, mix and drip into the sample hole, and read in 10 minutes. Results are valid within 15 minutes
Fast and easy to use
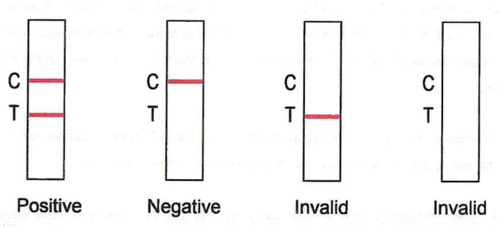
Positive: Red bands appear in both the test area (T) and the quality control area (C) , indicating a valid positive result. The color of the strip in the test area (T) can be dark or light, and all are positive results.
Negative: There is no red band appears in the test area (T), and a red band appears in the quality control area (C).
Invalid: If there is no band in the control area (C) , Regardless of whether there is a band in the test area (T), the test result is invalid. The test needs to be repeated.
Operation Suggestions
1. This product is a single-use in vitro diagnostic product, please use it within the validity period.
2. The test results of this product are for clinical reference, and should not be used as the only basis for clinical diagnosis and treatment. The clinical management of patients should be comprehensively considered in combination with their symptom.
s/signs, medical history, other laboratory tests, treatment responses, and epidemiol-ogy.
3. Users should read the Instructions for use carefully before operation, and carry out the test operation strictly in accordance with the kit instructions.
4. Wear disposable gloves when handling kits and samples, and wash hands horo ughly after handling.
5. All samples and wastes should be treated as sources of infection, The waste disposal is carried out in accordance with the relevant local regulations, please handle with care.
Operation Flow

Product inquiry
We will contact you within one working day. Please pay attention to your email.
Related Products



