Chlamydia Antigen Rapid Test Kit

This product is used for in vitro qualitative detection of Chlamydia (CHL) antigens in female cervical swabs and male urethral swabs. It is used for auxiliary diagnosis of Chlamydia (CHL) infection and is only used for in vitro diagnosis.
TEST TYPE: Female cervical swabs and male urethral swabs
RESULT IN: 15-30 minutes
Ordering Information
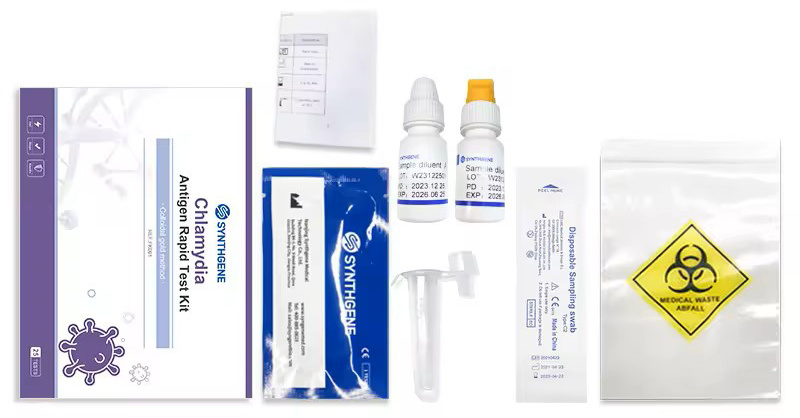
Specifications and performance
|
TEST TYPE |
female cervical swabs and male urethral swabs. |
|
CUT-OFF (DETECTION LEVEL) |
The enterprise limit of detection reference products (S1-S3) are tested, S1 is positive, S2 can be positive or negative, and S3 is negative. |
|
RESULTS IN |
15-30 minutes |
|
RELIABILITY |
Excellent (≈99%) |
|
MANUFACTURER |
SYNTHGENE |
About this test
Chlamydia is a prokaryotic microorganism with a unique developmental cycle and strict intracellular parasitism, which can pass through bacterial filters and it is one of the most prevalent sexually transmitted disease pathogens. Chlamydia infection has an average incubation period of 1-3 weeks. Male patients present with urethritis, often with dysuria or urethral discharge. Painful urination is less severe than gonorrhea, and sometimes only manifests as tingling and itching in the urethra. Urethral secretions are often serous or mucopurulent, thinner and less in volume. Female patients also have symptoms of urethral inflammation such as urgency and dysuria, but the main problem is endocervicitis. The cervix will have many symptoms: such as congestion, edema, easy bleeding to touch, increased yellow mucopurulent discharge, and lower abdominal discomfort, etc. However, there are also a considerable number of patients with mild symptoms or no clinical symptoms.
Common laboratory methods for diagnosing Chlamydia infection include the following:
①Microscopic examination: It is suitable for the examination of newborn eye conjunctival scraping blade; ②Cultivation method: Chlamydia cell culture positive;
③Antigen test: Enzyme-linked immunosorbent assay, direct immunofluorescence assay or immunochromatographic assay to detect positive Chlamydia antigen;
④Antibody test: Chlamydia IgM antibody titer increased in neonatal chlamydia pneumonia;
⑤Nucleic acid test: Chlamydia nucleic acid test is positive.
Quick and easy to use
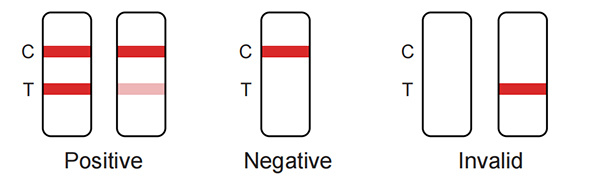
Positive: Red bands appear in both the test area(T) and the quality control area (C). The results show that the samples contain Chlamydia antigen.
Negative: There is no red band appears in the test area (T), and a red band appears in the quality control area (C). The results show that Chlamydia antigen is not detected in the samples.
Invalid: There is no red band in the quality control area (C), regardless of whether there is a red band in the test area (T), indicating the test result of the kit is invalid, and it is recommended to retest.
Product inquiry
We will contact you within one working day. Please pay attention to your email.
Related Products