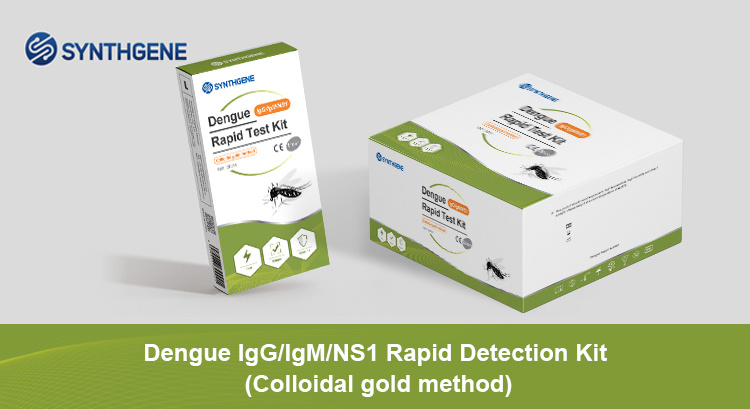
Dengue NS1 Rapid Test Kit

Dengue virus NS1 antigen detection can screen and assist in the diagnosis of early dengue infection within 15-30 minutes.
Test Type:whole blood (venous or fingertip blood), serum or plasma
Detection time: 15-30 minutes
Ordering Information
Specifications and performance
|
Test Type: |
whole blood (venous or fingertip blood), serum or plasma |
|
Detection time: |
15-30 minutes |
|
Reliability: |
Excellent |
|
Manufacturer: |
Nanjing Synthgene Medical Technology Co., Ltd. |
Operation process
About this test
This product is used for in vitro qualitative detection of dengue NSI antigen in
human whole blood/serum/plasma samples, and can be used for auxiliary diagnosis and screening of early clinical dengue virus infection.Dengue fever is caused by dengue virus (divided into 4 serotypes, DENV-1~4). It is an acute infectious disease transmitted by Aedes aegypti and Aedes albopictus, and is more common in tropical and subtropical regions. The clinical types can be divided into three types: dengue fever, dengue hemorrhagic fever and dengue shock syndrome.The product only needs use a disposable plastic straw to vertically drop 1 drop of fingertip blood sample or serum, plasma, whole blood sample into the sample well, and then drop 2 drops of sample diluent to start timing. Read the result at 10 minutes, and the result is valid within 30 minutes.
Fast and easy to use
1.This kit is only used for veterinary diagnosis, please operate in strict accordance with the instruction manual.
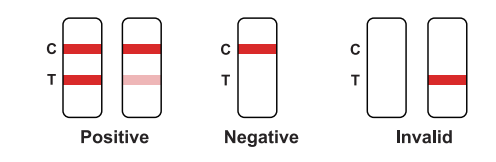
Positive: Red bands appear in both the test area (T) and the quality control area (C).The results show that the sample contains NSI antigen.
Negative: There is no red band appears in the test area (T), and a red band appears inthe quality control area (C). The results show that NS1 antigen is not detected in the sample.
Invalid: There is no red band in the quality control area (C), regardless of whetherthere is a red band in the test area (T), indicating the test result of the kit is invalid, and it is recommended to retest.
Note: Do not interpret the results in dim light.
lt is recommended not to make any medically related decisions without first consulting a healthcare provider.
Precautions
1. This product is only used for in vitro qualitative diagnosis, please use it within the validity period.
2. Please read the instruction manual carefully before use, and operate in strict accordance with the kit instructions.
3.The product is for one-time use. If the aluminum foil bag is found to be damaged.please do not use it. All samples and post-use products should be treated as infectious agents. It is carried out in accordance with the local national regulations on safe disposal of waste, safe disposal of infectious waste and safe disposal of infectious waste, please handle with caution.
4. If the refrigerated test reagents are used, it is recommended that they should be taken out of the refrigerator before the test and placed at room temperature before opening for use, otherwise the test results will be affected.
5. This kit contains animal-derived materials and has potential infectious risks, so try to avoid direct contact with the test pad.
6. Because this product is for visual interpretation, in order to ensure accurate results, please do not perform interpretation in dimly lit places.
7. If there is unknown drug interference or operational technical reasons, the result will be wrong; if the result is in doubt, please re-test.
8. There is a desiccant in the aluminum foil bag, which should not be taken orally; the sample diluent should not be taken orally.
9. The transportation of samples should comply with the local biosafety regulations.
Operation Flow
Product inquiry
We will contact you within one working day. Please pay attention to your email.
Related Products