HIV 1/2 Antibody Rapid Test Kit

The human immunodeficiency virus (HIV 1/2) antibody test can detect viral antibodies (HIV 1/2) in human whole blood/serum/AIDS in 10 minutes.
Test Type:Whole blood (fingertip blood/venous blood), serum, plasma.
Detection time: 10-30 minutes
Ordering Information
Specifications and performance
|
Test Type: |
Whole blood (fingertip blood/venous blood), serum, plasma. |
|
Limit of detection (LOD): |
0.2 NCU/ml |
|
Detection time: |
10-30 minutes |
|
Reliability: |
Excellent |
|
Manufacturer: |
Nanjing Synthgene Medical Technology Co., Ltd. |
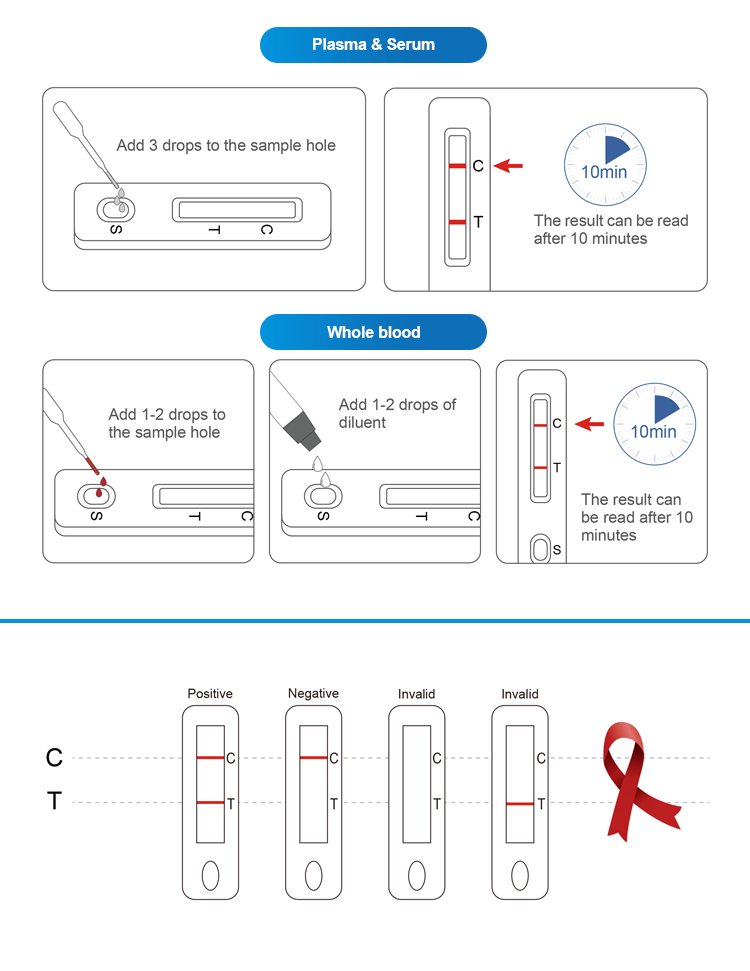
Operation process
About this test
This product is used for the in vitro qualitative detection of human immunodeficiencyvirus type 1 (HIV-1) antibodies in human urine.AIDS, namely acquired immunodeficiency syndrome, its pathogen is human immuno-deficiency virus (HIV), also known as AIDS virus, belonging to the retroviral familylentivirus genus.This product is for professional use only.Only 3 drops of the product are needed, the results will be observed in 10 minutes, and it will be effective within 30 minutes.
Fast and easy to use
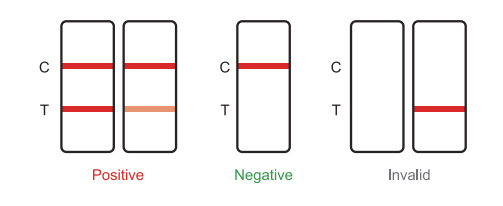
Positive: red bands appear in both the test area (T) and the quality control area (C) Indicates possible infection.
Negative: No red band appears in the detection area (T),Quality control area (C). This indicates that infection is not present and only occurs in rare cases.
Invalid: There is no red band in the quality control area (C), regardless of whether A red band appears in the detection area (T), indicating that the test result of the kit is invalid.
Operation Suggestions
1. This product is only used for in vitro qualitative diagnosis, please use it within the validity period.
2. Please read the instructions for use carefully before use, and operate in strict accord-ance with the kit instructions.
3. The product is for single-use. If the aluminum foil bag is found to be damaged, please do not use it. All samples and post-use products should be treated as infectious agents. Follow the local regulations in the safe disposal of waste and infectious waste, please handle with caution.
4. If the refrigerated test kits are used, it is recommended that they should be taken out of the refrigerator before the test and placed at room temperature before opening for use, otherwise the test results will be affected.
5. This kit contains animal-derived materials and has potential infectious risks, so try to avoid direct contact with the nitrocellulose membrane in test pad.
6. The sample diluent contains a small amount of preservatives, which may cause irritation to skin and eyes. If this solution comes into contact with skin or eyes, wash/rinse with plenty of water. If skin irritation or rash occurs, seek medical advice/attention.
7. It is not recommended for children, the elderly and pregnant women to purchase and self-test without a doctor's advice.
8. There is a desiccant in the aluminum foil bag, which should not be taken orally; the sample diluent should not be taken orally.
9. The transportation of whole blood/serum/plasma should meet the requirements of biosafety, and follow the local regulations.
Operation Flow
Product inquiry
We will contact you within one working day. Please pay attention to your email.
Related Products