HCV Antibody Rapid Test Kit

Hepatitis C virus antibody test can detect hepatitis C virus antibodies in human serum, plasma or whole blood samples within 10-30 minutes.
Test Type:Whole blood (fingertip blood/venous blood), serum, plasma.
Detection time: 10-30 minutes
Ordering Information
Specifications and performance
|
Test Type: |
Whole blood (fingertip blood/venous blood), serum, plasma. |
|
Detection time: |
10-30 minutes |
|
Limit of detection (LOD): |
0.2 NCU/ml |
|
Reliability: |
Excellent |
|
Manufacturer: |
Nanjing Synthgene Medical Technology Co., Ltd. |
Operation process
About this test
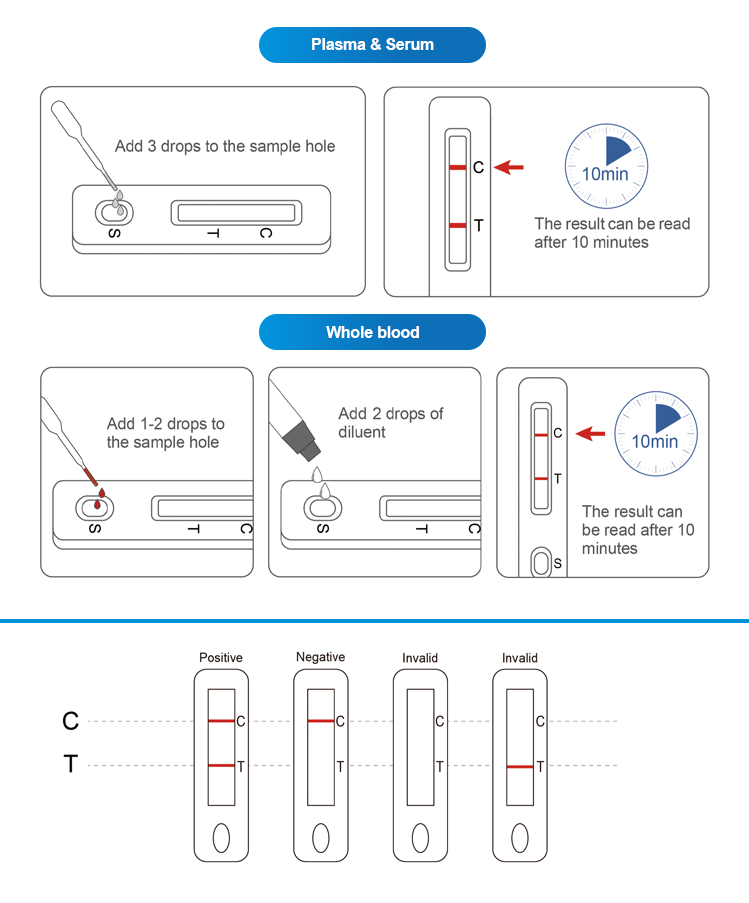
This product is used for the qualitative detection of hepatitis C virus (HCV) antibodies in human serum, plasma or whole blood samples. Hepatitis C virus is a single-stranded sense-stranded RNA virus with an envelope. It is a non-A, non-B hepatitis virus that mainly causes parenteral transmission.HCV is mainly spread through blood sources, but also through other methods such as vertical transmission frommotherto child, daily family contact, and sexual transmission.The product only requires three drops of human plasma/spine (approximately 75ul) and two drop of human whole blood (fingertip blood or venous blood) (approximately 50ul). The results will be observed in 10 minutes and will be effective within 30 minutes.
Fast and easy to use
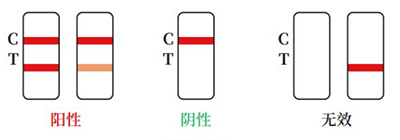
Positive:Red bands appear in both the test area (T) and the control area (C). The results show that the sample contains HCVAb.
Negative:There is no red band in the test area (T), and a red band appears in the control area (C).The results show that HCVAb is not detected in the sample.
Invalid:No red band appears in the control area (C), regardless of whether there is a red band in the test area (T), the test pad is judged to be invalid, and it is recommended to perform aretest.
Operation Suggestions
1. This product is a disposable in vitro diagnostic product, please use it within the validity period.
2. Please read the instruction manual carefully before use, the inspection personnel mustbe professionally trained to operate, and the test operation should be carried out in strict accordance with the instructions of the kit.
3. If you find that the aluminum foil bag packaging is damaged, please do not use it. Please use it as soon as possible after opening the aluminum foil bag. Do not conduct experiments in excessively high temperature or humidity and excessively dry environ- ments. The kits stored at low temperatures need to be restored to room temperature before opening to avoid moisture absorption.
4. If the test result is negative and there are clinical symptoms, other clinical methods can be recommended for testing. A negative result does not rule out the possibility of infection.
5. The test of the sample must be carried out in a specific environment, and the blood sample contacted during the test should be operated in accordance with the inspection procedures of the infectious disease laboratory.
6. Temperature has a great influence on the test results.
7. Samples containing high concentrations of rheumatoid factors or heterophilic antibodies may cause false positive results.
8. It should be kept clean, and pollutants should be treated as waste. The waste treatment is carried out in accordance with the local regulations for the safe disposal of waste and the infectious waste. Please handle with care.
Operation Flow
Product inquiry
We will contact you within one working day. Please pay attention to your email.
Related Products