FluA/FluB Antigen Rapid Test Kit

Influenza A/B test can detect infection with influenza A/B virus in 10-30 minutes.
Test Type:Nasal swab+Oropharyngeal swab
Detection time: 15-30 minutes
Ordering Information
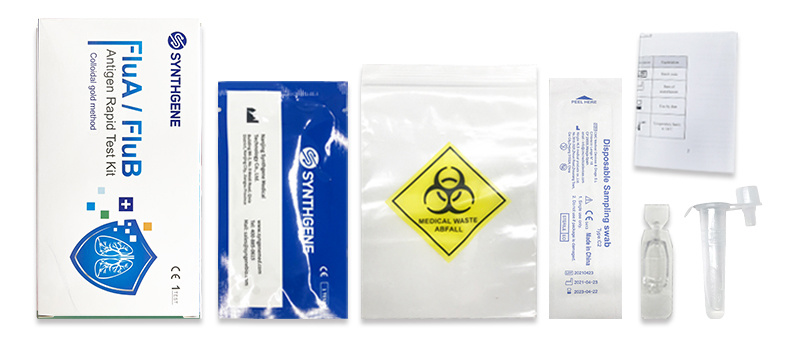
Specifications and performance
|
Test Type: |
Nasal swab+Oropharyngeal swab |
|
Detection time: |
15-30 minutes |
|
Reliability: |
Excellent |
|
Manufacturer: |
Nanjing Synthgene Medical Technology Co., Ltd. |
About this test
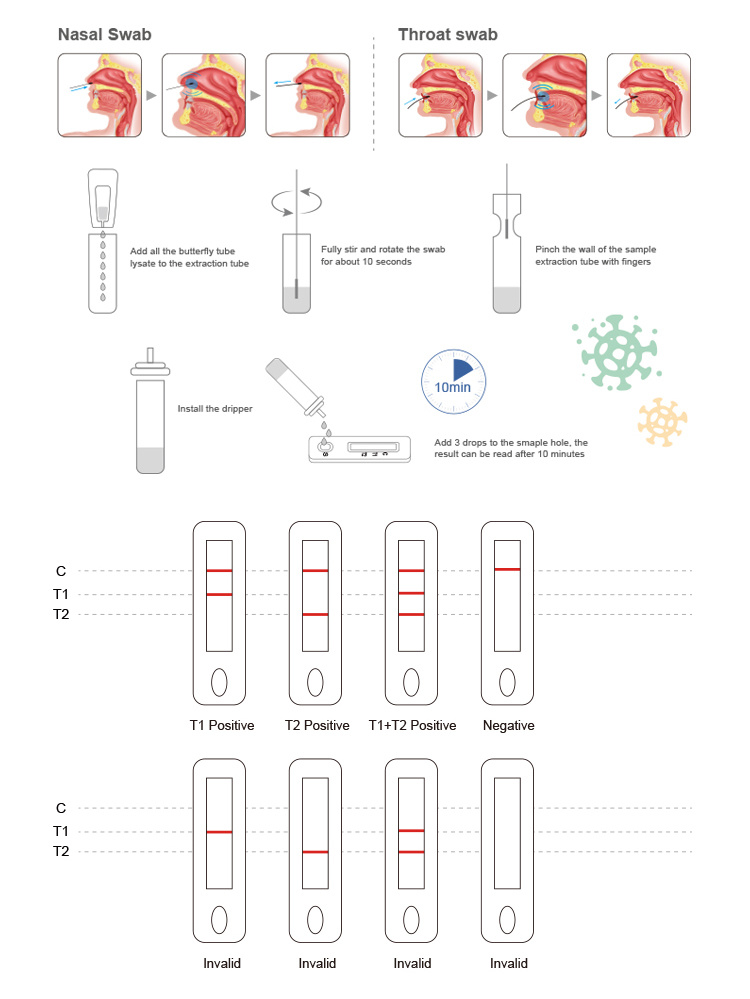
FluA/FluB Antigen Rapid Test Kit (Colloidal gold method) is used for in vitro qualitative detection of influenza A/B virus antigens in human nasal swab and oropharyngeal swab samples.Influenza virus is mainly transmitted by air droplets, often causing fever, fatigue, muscle aches and mild to moderate respiratory symptoms. In severe cases, it can cause pneumonia, myocarditis and heart failure.This product can be used for the auxiliary diagnosis of influenza. The antigen detection reagents for each subtype of influenza A virus can also be used to distinguish seasonal influenza viruses from new influenza A viruses, and to obtain epidemiological information on influenza outbreaks. Clinically, it is mainly used for the differential diagnosis of influenza A/B. This product is suitable not only for professional medical personnel to test in medical institutions, but also for consumer self-test.The product only requires collecting nasal swabs or oropharyngeal swabs, immersing them in the sample solution, adding 3 drops to the sampling hole, and the results will be available in 15 minutes, and the results will be valid within 30 minutes.
Fast and easy to use
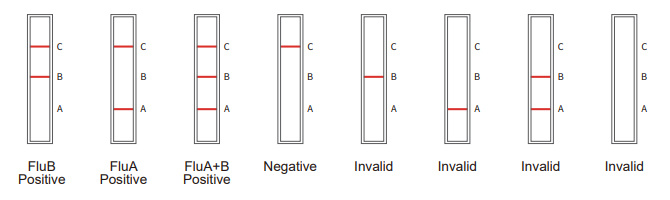
Positive:For Fl A: red bands appear in both the test line (A) and the contro linc (C)
For Flu B: red bands appear in both the test line (B) and the control line (C).
For Flu A+Flu B: red bands appear in the test lines (A and B) and the controi line (C).
Negative:There is no red band in the test line (A and B), and a red band in the control line (C).
Invalid:No red band appcars in the control line (C), regardless of whether there is a red band in the tes! line (A or
B), the test result is deemed invalid and retesting is reccrumended.
Operation Suggestions
1. This product is only used for in vitro qualitative diagnosis, pleasc use it within thc validity period.
2. Piease read the instructions for use carefully before use and strictly follow the the iustructjons for use to operation.
3. The product is for single-use. If the aluminum foil bag packaging is found to be damaged, please do not use iL All samples and post-usc products should be treated as infectious agents and handled with caufion.
4. If using test kits stcred in the regerator, it is recommended to take them out of the refrigerator before testing and let them return to room tempcrature before opening them for use. Otherwisc, the test results wiil be aftccted.
5. Compared with adults, chiidren arc more likely to spread the virus over a larger area and for a longer period of time. Therefore, thc sensitivity of detcction in children may be higher than that in adults.
6. For influenza A virus or subtype dctection kits, when the antibody used is monoclonal in nature, small changcs in the antigenic epitope caused by smail-scale inutations in the nucleotidc sequence mmay lead to false negativc results or a reduction in the analytical sensitivity of the kit.
7. When handling Sampics, do not bend the swab forcefully to avoid breaking it. Please ensure that an appropriate amouni of sampie is used for testing. Too tnuch or too littlc sample may lead to biased results
8. The lysis buffcr contains a small amount of preservatves, which may cause irrilation to the skin and eyes. If the buffer comes into contact with skin or eyes, wash/flush with picnty of water, If skin irritation or rash occurs, seek medical care/aftendance.
9,Inconsistent or erroneous resuits may be caused by improper teclnical or step operations, samplc contamination, or the presence of drugs that interfere with the test.
10. There is a desiccant in the aluminum foil bag and must not be taken orally, and the lysis bufier must not be taken orally.
Operation Flow
Product inquiry
We will contact you within one working day. Please pay attention to your email.
Related Products