COVID-19/Influenza A+B/RSV Antigen Combo Rapid Test Kit

This product is used for the qualitative detection of COVID-19 (2019-nCoV), influenza A(FluA), influenza B(FluB) and respiratory syncytial virus(RSV) antigens in human nasal swabs and nasopharyngeal swabs in vitro.
TEST TYPE: Nasal Swab/Oropharyngeal Swab
RESULT IN: 15-20 minutes
Specifications and performance
|
TEST TYPE |
Nasal Swab/Oropharyngeal Swab |
|
CUT-OFF (DETECTION LEVEL) |
Test the enterprise minimum detection limit reference products S1, S2, S3, S4, S5, and S6 for detection. S1-S4 should be positive, S5can be positive or negative, and S6 should be negative. |
|
RESULTS IN |
15-20 minutes |
|
RELIABILITY |
Excellent (≈99%) |
|
MANUFACTURER |
SYNTHGENE |
About this test
This product uses colloidal gold immunochromatography combined with double-antibody sandwich principle to detect novel coronavirus N antigen, influenza A virus antigen, influenza B virus antigen and respiratory syncytial virus antigen in human nasal swab and nasopharyngeal swab samples.
According to a recent survey of clinicians in many fever clinics, under the COVID-19 epidemic, it is often necessary to identify three common respiratory infections,
common cold, flu, and novel coronavirus. The clinical symptoms of the three in the early stage of infection are highly similar, but the treatment options are different. The
common cold can be caused by rhinovirus, coronavirus, parainfluenza virus, syncytial virus and other viral infections. The prognosis of the patient is good. The complications caused by novel coronavirus infection and influenza infection in high-risk groups are more similar, which can cause systemic symptoms and even lead to
death. Therefore, clinically, there are clear diagnosis and treatment plans for the infection of these two viruses. In the post-epidemic period when the epidemic spreads,
especially in the traditional high-influenza season, it is necessary to conduct joint detection of influenza virus, syncytial virus and novel coronavirus, timely differential
diagnosis, identify co-infection, and take targeted treatment measures as soon as possible.
This product does not require any equipment, and the entire detection process only takes 15-20 minutes. Simple operation, fast, accurate, high sensitivity, etc., especially
suitable for screening of suspected patients, hospital laboratory, special departments (blood transfusion, preoperative, emergency department, etc.), disease control
system (epidemic prevention station), entry-exit quarantine and other places (medical examination).
Quick and easy to use
COVID-19 test results:
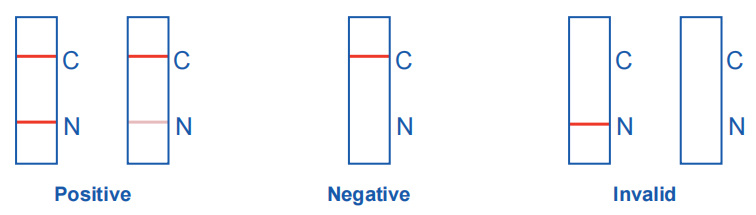
Positive: Two purple-red bands appear. One band is located in the detection area (N), and the other one is located in the quality control area (C).
Negative: Only a purple-red band appears in the quality control area (C), and no band appears in the detection area (N).
Invalid: There is no purple-red band in the quality control area (C), no matter whether there is a band in the detection area (N). The result is invalid, and it is recommended to retest.
Influenza A and Influenza B virus test results:
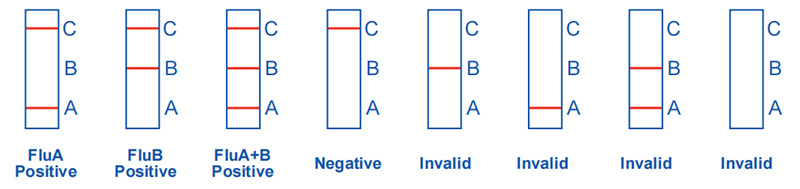
Positive: Influenza A positive: There are purple-red bands in both the detection area (A) and the quality control area (C).
Influenza B positive: There are purple-red bands in both the detection area (B) and the quality control area (C).
Influenza A and influenza B positive: Purple-red bands appear in both the detection area (A and B) and the quality control area (C).
Negative: Only a purple-red band appears in the quality control area (C), and no band appears in the detection area (A and B).
Invalid: There is no purple-red band in the quality control area (C), no matter whether there is a red band in the detection area (A and B). The result is invalid, and it is recommended to retest.
Respiratory syncytial virus test results:
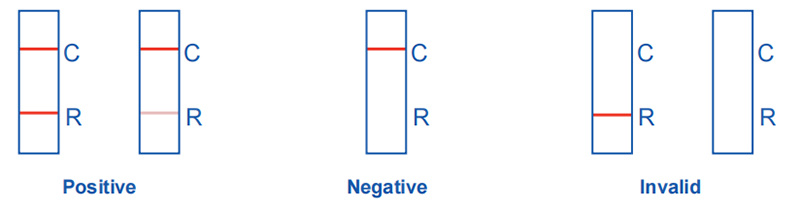
Positive: Two purple-red bands appear. One band is located in the detection area (R), and the other one is located in the quality control area (C).
Negative: Only a purple-red band appears in the quality control area (C), and no band appears in the detection area (R).
Invalid: There is no purple-red band in the quality control area (C), no matter whether there is a band in the detection area (R). The result is invalid, and it is recommended to retest.
Product inquiry
We will contact you within one working day. Please pay attention to your email.
Related Products