SARS-CoV-2 Virus Neutralization Test kit

This product is used for in vitro qualitative detection of novel coronavirus (SARS-COV-2) neutralizing antibodies in human serum/plasma/whole blood/fingertip blood.
TEST TYPE: Serum/Plasma/Whole Blood/Fingertip Blood
RESULT IN: 10-15 minutes
Specifications and performance
|
TEST TYPE |
serum/plasma/whole blood/fingertip blood |
|
CUT-OFF (DETECTION LEVEL) |
The comparison experiment is carried out with the enterprise reference product, L1 is the sensitivity 1 of the reference product, the test result is positive, L2 is the sensitivity 2 of the reference product, the test result can be positive or negative, and L3 is the enterprise reference product. The sensitivity of the product is 3, the test result is negative, and L1 is the detection limit of the enterprise reference product. |
|
RESULTS IN |
10-15 minutes |
|
RELIABILITY |
Excellent (≈99%) |
|
MANUFACTURER |
SYNTHGENE |
About this test
The 2019-nCoV neutralizing antibody is a protective antibody produced by the human body after being vaccinated with the novel coronavirus vaccine or infected with the novel coronavirus. Neutralizing antibodies have the function of recognizing the surface S-RBD protein of the novel coronavirus, blocking the binding of S-RBD to the cell surface specific receptor (ACE2), and exerting an antiviral effect. Whether neutralizing antibodies are produced and the amount or titer produced is an important indicator of the immune protection effect after vaccination with the novel coronavirus vaccine, and it is also an important basis for vaccine evaluation and quality control. One of the important indicators of its cure evaluation, it is helpful for the screening of novel coronavirus vaccinated population and the judgment of the effect after vaccination.
Quick and easy to use
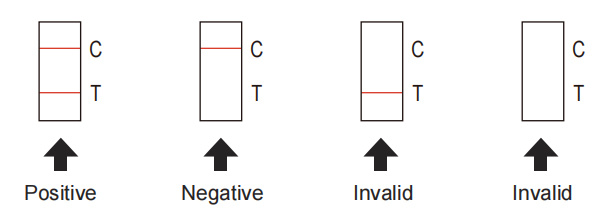
Positive: Two red or purple bands appear. One is in the detection area (T) and the other is in the quality control area (C). The color of the band in the detection area (T) can be dark or light, and all are positive results. Positive results indicate that the sample contains novel coronavirus neutralizing antibodies.
Negative: Only a red or purple band appears in the control area (C), and no band appears in the detection area (T). Negative results indicate that novel coronavirus neutralizing antibody is not detected in the sample.
Invalid: No red or purple band appears in the control area (C), regardless of whether there is a band in the detection area (T). If the result is invalid, the sample needs to be re-tested.
Product inquiry
We will contact you within one working day. Please pay attention to your email.
Related Products