COVID-19/Influenza A+B Antigen Combo Rapid Test Kit

This product is used for in vitro qualitative detection of novel coronavirus (COVID-19) and influenza A/B virus antigens in human nasal swab and oropharyngeal swab samples.
TEST TYPE: Nasal Swab/Oropharyngeal Swab
RESULT IN: 10-15 minutes
Specifications and performance
|
TEST TYPE |
Nasal Swab/Oropharyngeal Swab |
|
CUT-OFF (DETECTION LEVEL) |
Use the enterprise minimum detection limit reference products (S1-S6) for testing, S1-S4 are all positive, S5, S6 are not required. |
|
RESULTS IN |
10-15 minutes |
|
RELIABILITY |
Excellent (≈99%) |
|
MANUFACTURER |
SYNTHGENE |
About this test
This product is a rapid diagnostic product developed based on immunocolloidal gold technology and immunochromatography technology. The colloidal gold-labeled immunochromatographic detection test strip of the novel coronavirus N protein antigen and the colloidal gold-labeled immunochromatographic detection test strip of the influenza A/B virus antigen are assembled into a dual detection kit. The double antibody sandwich method is used to qualitatively determine the novel coronavirus antigen and influenza A/B virus antigen in nasal swab and oropharyngeal swab samples. After the sample is added to each sample hole of the detection area, it migrates to the chromatography area by capillary action, and the colloidal gold-labeled particles are enriched due to the antigen-antibody reaction, forming or not forming a colloidal gold-red reaction band, thus realizing the detection of novel coronavirus (2019-nCoV) and influenza A/B virus antigens, and determination of presence or absence.
Quick and easy to use
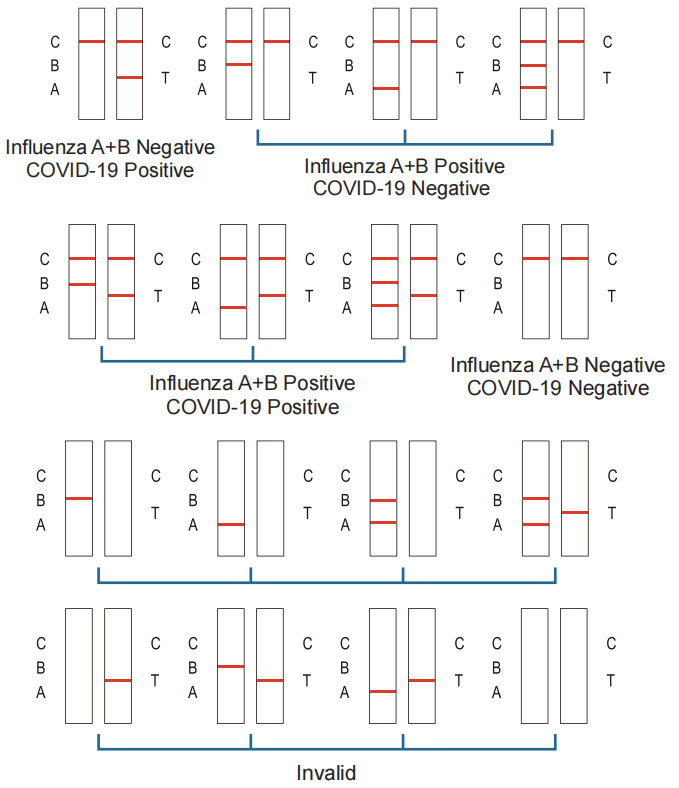
Positive:COVID-19 Test: Purple-red bands appear in both the detection area (T) and the quality control area (C). The results show the sample contains SARS-CoV-2.
Influenza A/B virus Test: Red bands appear in both the detection area (A) and the quality control area (C). The results show the samples contains influenza A virus antigens. Red bands appeared in both the detection area (B) and the quality control area (C). The results showed that: the sample contains influenza B virus antigen. Red bands appeared in both the detection area (A and B) and the quality control area (C).The results show it may be a mixed infection of influenza A virus and influenza B virus. Microbiological culture is recommended for individuals with symptoms of infection who have negative test results to determine whether they are infected with the virus. If the culture result is different from the test result, another sample should be taken for microbial culture review.
Negative:COVID-19 Test: There is no purple-red band in the detection area (T), and a purple-red band appears in the quality control area (C). The results show that SARS-CoV-2 is not detected in the sample. Influenza A/B virus Test: There is no red band in the detection area (A and B), and a red band appears in the quality control area (C). The results show that influenza A and influenza B antigens are not detected in the sample.
Invalid: There is no red band in the quality control area (C), regardless of whether there is a red band in the detection area (T, A, or B), the test pad is judged to be invalid, and it is recommended to retest. If there are still problems, stop using the batch of products immediately and contact the local supplier.
Product inquiry
We will contact you within one working day. Please pay attention to your email.
Related Products